The question “is sodium molybdate ionic or covalent” aims to understand the type of chemical bonding present in sodium molybdate. We can rephrase it as: “Does sodium molybdate exhibit ionic or covalent bonding, and what factors determine its bonding nature?”
Is Sodium Molybdate Ionic or Covalent? Unveiling the Bonding Nature
Related Encyclopedia
Related Products More >
-
- 7757-82-6
- CNY equest For Quotation
-
- 10124-56-8
- CNY equest For Quotation
-
- 7758-19-2
- CNY equest For Quotation
-
- 2893-78-9
- CNY equest For Quotation
-
- 55310-46-8
- CNY equest For Quotation
-
- 136-30-1 DibutylCarbamodithioic acid sodium salt
- CNY equest For Quotation
-
- CNY equest For Quotation
-
- CNY equest For Quotation



 沪ICP备2021018848号-5
沪ICP备2021018848号-5

In general, compounds formed between metals and non - metals or poly - atomic ions tend to be ionic.Electron Transfer: Sodium has one valence electron. It readily loses this electron to achieve a stable noble - gas configuration.
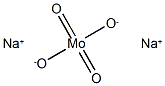
The molybdate ion MoO4^2- is a poly - atomic ion that has a net negative charge. Two sodium atoms each donate one electron to the molybdate ion. The electrostatic attraction between the positively charged sodium ions Na^+ and the negatively charged molybdate ion MoO4^2 - forms the ionic bond.
Physical Properties: Ionic compounds typically have high melting and boiling points, are brittle, and conduct electricity when molten or in aqueous solution. Sodium molybdate has a high melting point (about 687^circ C ), and its aqueous solutions can conduct electricity, which are characteristic properties of ionic compounds.Case Study: Use in the Chemical IndustrySodium molybdate is widely used in the corrosion inhibition of metal surfaces. For example, in the oil and gas industry, pipelines are often made of steel, which is prone to corrosion in the presence of water and oxygen.
Sodium molybdate can be added to the water - based fluids in the pipelines.
When dissolved in water, sodium molybdate dissociates into Na^+ and MoO4^2 - ions. The molybdate ions can form a protective layer on the steel surface. This layer acts as a barrier, preventing the oxidation of iron in the steel and thus reducing corrosion. The ionic nature of sodium molybdate allows it to dissolve easily in water, enabling it to be effectively transported and used in these applications.
Operational Considerations and SafetyHandling: When handling sodium molybdate, wear appropriate personal protective equipment (PPE), such as gloves, goggles, and a lab coat. Although it is not highly toxic, it can cause skin and eye irritation.Storage: Store sodium molybdate in a cool, dry place.
Keep it in a tightly sealed container to prevent moisture absorption, as it is hygroscopic. Moisture can cause caking, which may affect its performance in some applications.Disposal: Follow local regulations for the disposal of sodium molybdate.
Do not dispose of it in regular trash or pour it down the drain without proper treatment, as it can be harmful to the environment.Reaction with Other Substances: Be aware of potential reactions with other chemicals. For example, it may react with strong acids to form molybdic acid and sodium salts. Avoid contact with incompatible substances to prevent unwanted chemical reactions.
Its chemical formula is Na₂MoO₄, which consists of sodium ions (Na⁺) and a molybdate ion (MoO₄²⁻). In sodium molybdate, the bonding between the sodium ions and the molybdate ion is ionic. Sodium, a metal, loses one electron to form a Na⁺ ion, while the molybdate ion is a negatively charged polyatomic ion made of molybdenum and oxygen atoms.
The connection between the Na⁺ ions and the MoO₄²⁻ ion is based on electrostatic attraction — the force between oppositely charged particles — which is characteristic of ionic bonds.
However, inside the molybdate ion (MoO₄²⁻) itself, the molybdenum (Mo) atom and the oxygen (O) atoms are connected by covalent bonds. The molybdenum and oxygen atoms share electrons to form the structure of the MoO₄²⁻ ion.
In short:
The bond between sodium (Na⁺) and molybdate (MoO₄²⁻) is ionic.
The bonds within the molybdate ion (between Mo and O atoms) are covalent.
Therefore, sodium molybdate as a whole is considered an ionic compound because it is composed of ions held together by ionic bonds.
Explanation: To understand why sodium molybdate is classified as ionic, we must examine the types of bonds formed between its constituent atoms. Sodium (Na) is a metal, while molybdenum (Mo) and oxygen (O) are non-metals. Ionic compounds typically form between metals and non-metals due to the significant difference in their electronegativities.
In sodium molybdate, sodium donates electrons to form positively charged ions (Na⁺), while the molybdenum and oxygen atoms form negatively charged ions (MoO₄²⁻). The electrostatic attraction between these oppositely charged ions results in the formation of an ionic bond. This bond is strong and typically leads to the formation of crystalline structures, which is characteristic of ionic compounds.
Moreover, sodium molybdate readily dissolves in water, a characteristic feature of ionic compounds. When dissolved, it dissociates into its constituent ions, Na⁺ and MoO₄²⁻, which can conduct electricity in solution. This behavior further confirms its ionic nature.
Reasons Behind the Ionic Nature
The ionic nature of sodium molybdate can be attributed to several factors:
Electronegativity Difference: Sodium has a relatively low electronegativity (approximately 0.93), while molybdenum and oxygen have higher electronegativities (approximately 1.88 for Mo and 3.44 for O). This significant difference in electronegativity facilitates the transfer of electrons from sodium to the molybdate ion, resulting in the formation of ions.
Formation of Ions: Sodium, being a Group 1 element, has one valence electron that it readily loses to achieve a stable electron configuration. Molybdenum, on the other hand, can form multiple oxidation states, allowing it to accept electrons and form the molybdate ion (MoO₄²⁻). The combination of these ions leads to the formation of an ionic lattice.
Physical Properties: The physical properties of sodium molybdate, such as its high melting point and solubility in water, are consistent with those of ionic compounds. These properties arise from the strong electrostatic forces between the ions in the lattice.
Case Study: Application in Agriculture
One of the most significant applications of sodium molybdate is in the agricultural industry, where it is used as a fertilizer. Molybdenum is an essential micronutrient for plants, playing a crucial role in nitrogen fixation and the metabolism of nitrogen-containing compounds. Sodium molybdate is often added to fertilizers to ensure that plants receive an adequate supply of molybdenum.
Example: In regions where soil molybdenum levels are low, the application of sodium molybdate can significantly enhance crop yields. For instance, leguminous plants, which rely on nitrogen-fixing bacteria in their root nodules, benefit greatly from molybdenum supplementation. The presence of sodium molybdate in the soil helps these bacteria function more efficiently, leading to improved nitrogen fixation and ultimately better plant growth.
Case Study: Industrial Applications
In addition to its agricultural uses, sodium molybdate is also employed in various industrial processes. It serves as a corrosion inhibitor in cooling water systems, protecting metal surfaces from rust and degradation. The molybdate ion forms a protective layer on metal surfaces, preventing corrosion caused by oxygen and other corrosive agents.
Example: In power plants, where cooling water systems are critical for maintaining efficient operation, sodium molybdate is added to the water to inhibit corrosion. This not only extends the lifespan of the equipment but also reduces maintenance costs, making sodium molybdate an invaluable compound in industrial settings.
Precautions During Operation or Use
While sodium molybdate is generally safe to handle, there are several precautions to keep in mind:
Personal Protective Equipment (PPE): Always wear appropriate PPE, including gloves and safety goggles, when handling sodium molybdate to prevent skin and eye irritation.
Ventilation: Work in a well-ventilated area to avoid inhalation of any dust or fumes that may be generated during handling or mixing.
Proper Storage: Store sodium molybdate in a cool, dry place, away from incompatible substances. Ensure that containers are tightly sealed to prevent moisture from entering, which could lead to clumping or degradation.
Disposal: Dispose of any waste containing sodium molybdate in accordance with local regulations. Do not pour it down the drain, as it can harm aquatic life.
Spill Management: In case of a spill, clean it up immediately using absorbent materials. Avoid direct contact with skin and thoroughly wash affected areas with water.