Hello, I'd like to ask a question. Is oxalic acid polar, non-polar, or ionic? Please explain the reason and how its polarity, non-polarity, or ionic characteristic affects its solubility and dissolution behavior in different solvents. Also, how do external conditions like temperature and pressure alter oxalic acid's tendency to exhibit polar, non-polar, or ionic states? Compared to other organic acids with similar structures, what unique characteristics and differences does oxalic acid have in terms of its polarity, non-polarity, or ionic nature?
Is Oxalic Acid Polar, Nonpolar, or Ionic?
Related Encyclopedia
Related Products More >
-
- 144-62-7
- USD 16.0000
- 25kg
-
- 996-98-5
- equest For Quotation
- 25kg/drum
-
- 856335-90-5
- equest For Quotation
- 25kg/ea
-
- 144-62-7
- equest For Quotation
- Bag
-
- 144-62-7
- equest For Quotation
- 25
-
- 144-62-7
- equest For Quotation
- 25kg/Drum
-
- 144-62-7
- equest For Quotation
- 1Kg/Foil bag,25Kg/Drum
-
- 144-62-7
- equest For Quotation
- 25kg



 沪ICP备2021018848号-5
沪ICP备2021018848号-5

Reason:
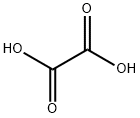
Oxalic acid consists of two carboxyl groups (–COOH) connected by a carbon-carbon single bond. The electronegativity difference between carbon (C), oxygen (O), and hydrogen (H) atoms creates polar covalent bonds within the molecule. Additionally, the carboxyl groups can donate protons (H⁺) in aqueous solutions, leading to partial ionization and the formation of oxalate ions (C₂O₄²⁻).
Impact of Polarity and Ionic Characteristics on Solubility:
In Polar Solvents (e.g., Water):
Oxalic acid dissolves readily due to hydrogen bonding and dipole-dipole interactions between its polar carboxyl groups and the polar solvent molecules.
It partially ionizes in water, forming hydrogen ions (H⁺) and oxalate ions (C₂O₄²⁻), which further enhances solubility.
In Nonpolar Solvents (e.g., Hexane):
Oxalic acid is poorly soluble because nonpolar solvents cannot effectively interact with its polar carboxyl groups.
No ionization occurs in nonpolar solvents, as they lack the polarity required to stabilize ions.
In Alcohols (e.g., Ethanol):
Oxalic acid exhibits intermediate solubility, depending on the alcohol's polarity. Short-chain alcohols (e.g., methanol, ethanol) dissolve oxalic acid well due to hydrogen bonding, while long-chain alcohols (e.g., octanol) show reduced solubility.
Effect of Temperature and Pressure:
Temperature:
Higher temperatures generally increase the solubility of oxalic acid in polar solvents by providing energy to overcome intermolecular forces.
Elevated temperatures can also enhance ionization in aqueous solutions, as dissociation is endothermic.
Pressure:
Pressure has a minimal effect on the solubility of oxalic acid in liquids, as it primarily impacts gas-liquid systems.
However, high pressure can slightly increase solubility in some cases by compressing the solvent and improving interactions.
Comparison with Similar Organic Acids:
Acetic Acid (CH₃COOH):
Oxalic acid has two carboxyl groups, making it more polar and acidic than acetic acid, which has only one carboxyl group.
This difference enhances oxalic acid's solubility in water and its ability to form stable metal complexes.
Citric Acid (C₆H₈O₇):
Citric acid has three carboxyl groups, making it more polar and water-soluble than oxalic acid.
However, oxalic acid's smaller size and simpler structure allow it to form stronger hydrogen bonds and more stable metal chelates.
Formic Acid (HCOOH):
Formic acid is simpler and less polar than oxalic acid, with only one carboxyl group.
Oxalic acid's additional carboxyl group gives it higher acidity and reactivity in chemical reactions.
However, oxalic acid can exhibit ionic characteristics under certain conditions, such as when it dissociates in water, releasing hydrogen ions (H+), thereby forming oxalate ions (C2O4^2-) and water-soluble salts with cations. Yet, fundamentally, the molecule itself is considered polar rather than ionic because of the covalent bonds holding it together internally.
In practical applications, oxalic acid finds use in various industries including cleaning products, particularly for rust removal due to its ability to form soluble complexes with iron oxides. It is also used in bleaching and as a reducing agent in chemical processes. When discussing whether oxalic acid is polar, non-polar, or ionic, it's important to consider both its inherent molecular polarity and its behavior in different environments. Understanding these properties is crucial for predicting its reactivity and compatibility in numerous applications, from household cleaning to industrial manufacturing processes. This dual nature of being primarily polar but capable of ionic interactions highlights the versatility of oxalic acid in chemistry.
Influence on solubility:
In polar solvents like water, the polar nature of oxalic acid allows it to dissolve well through dipole - dipole interactions and hydrogen bonding. The water molecules can interact with the polar groups of oxalic acid, breaking the intermolecular forces in the solid and solvating the molecules. In non - polar solvents, oxalic acid has low solubility because there are no favorable intermolecular forces between the non - polar solvent and the polar oxalic acid molecules. When it ionizes in solution, the ions are more soluble in polar solvents due to the strong ion - dipole interactions.
Effect of external conditions:
An increase in temperature generally promotes the ionization of oxalic acid. Higher temperatures provide more energy for the proton - transfer process, increasing the tendency to form ions. Pressure has a relatively minor effect on the polarity or ionization of oxalic acid under normal conditions. However, extremely high pressures may affect the intermolecular distances and interactions, potentially influencing the ionization equilibrium to a small extent.
Differences from other organic acids:
Compared to some monocarboxylic acids, oxalic acid has two carboxyl groups in close proximity. This leads to stronger intermolecular forces and a higher degree of ionization. It can form more extensive hydrogen - bonding networks and has a higher acid strength. For example, acetic acid is a monocarboxylic acid and is less acidic and has weaker intermolecular forces compared to oxalic acid.